PRACTICE ESSENTIALS
Persistent Pulmonary Hypertension of Newborn
Persistent pulmonary hypertension of the newborn (PPHN) is defined as the failure of the normal circulatory transition that occurs after birth. It is a syndrome characterized by marked pulmonary hypertension that causes hypoxemia secondary to right-to-left shunting of blood.
Signs and symptomsPPHN is often associated with the following signs and symptoms of perinatal distress:
- Asphyxia
- Tachypnea, respiratory distress
- Loud, single second heart sound (S2) or a harsh systolic murmur (secondary to tricuspid regurgitation)
- Low Apgar scores
- Meconium staining
- Cyanosis; poor cardiac function and perfusion
- Systemic hypotension
- Symptoms of shock
Idiopathic persistent pulmonary hypertension of the newborn can present without signs of acute perinatal distress. Marked lability in oxygenation is frequently part of the clinical history. One cause of idiopathic PPHN is constriction, or premature closure of the ductus arteriosus in utero, which can occur after exposure to nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, ibuprofen, naproxen) during the third trimester.
DiagnosisSuspect PPHN whenever the level of hypoxemia is out of proportion to the level of pulmonary disease. Clinically, PPHN is most often recognized in term or near-term neonates, but it can occur in premature neonates, albeit infrequently.
In contrast to adult primary pulmonary hypertension, the newborn syndrome is not defined by a specific pressure of the pulmonary circulation. The diagnosis is confirmed regardless of the pulmonary arterial pressure, as long as it is accompanied by right-to-left shunt and absence of congenital heart disease. [1]
Echocardiography is considered the most reliable noninvasive test to establish the diagnosis, assess cardiac function, and exclude associated structural heart disease.
Differential DiagnosisThe differential diagnosis for persistent pulmonary hypertension of the newborn (PPHN) includes the following:
- Congenital heart disease, including transposition of the great arteries, [24] total anomalous pulmonary venous connection, tricuspid atresia, and pulmonary atresia with intact ventricular septum
- Primary parenchymal lung disease such as bronchopulmonary dysplasia (BPD),[25] neonatal pneumonia, respiratory distress syndrome, pulmonary sequestration, and pulmonary hypoplasia resulting in hypercarbia and respiratory acidosis
- Sepsis
- Alveolar capillary dysplasia
- Surfactant protein B deficiency
- Metabolic acidosis of any etiology
- Arterial blood gas levels (through indwelling line): To assess the pH, partial pressure of carbon dioxide in arterial gas (PaCO 2), and the partial pressure of oxygen (PaO 2)
- Complete blood count with differential: To evaluate for high hematocrit level (polycythemia and hyperviscosity syndrome may lead to or exacerbate PPHN); to determine whether an underlying sepsis or pneumonia is present
- Coagulation studies (eg, platelet count, prothrombin time, partial thromboplastin time, international normalized ratio): To assess for coagulopathy (increased disease severity)
- Serum electrolytes (eg, calcium) and glucose levels
- Preductal and postductal oxygen saturation measurements via pulse oximetry to assess for differential cyanosis
- Chest radiography: To assess for presence of underlying parenchymal lung disease (eg, meconium aspiration syndrome, pneumonia, surfactant deficiency) and/or to exclude underlying disorders (eg, congenital diaphragmatic hernia); see the image below

Meconium aspiration. Radiograph obtained shortly after birth shows ill-defined, predominantly perihilar opacities in the lungs; these are more severe on the right than on the left. The lungs are hyperexpanded. The neonate's heart size is within normal limits.
- Echocardiography: To screen and assist in making the diagnosis of PPHN and to rule out total anomalous pulmonary venous return before considering administration of extracorporeal membrane oxygenation therapy (ECMO)
- Echocardiography with Doppler flow: To assess presence/direction of the intracardiac shunt at the ductus arteriosus and foramen ovale, as well as estimate the pulmonary arterial systolic/diastolic pressures
- Cranial ultrasonography: To assess for intraventricular bleeding and for peripheral areas of hemorrhage or infarct if ECMO is necessary
- Cranial ultrasonography with Doppler flow: To assess whether a nonhemorrhagic infarct is present
- Brain computed tomography scanning or magnetic resonance imaging: To evaluate for central nervous system injury
- Cardiac catheterization: Rarely utilized to exclude congenital heart disease (eg, obstructed anomalous pulmonary venous return, pulmonary vein stenosis) because echocardiographic findings are typically diagnostic
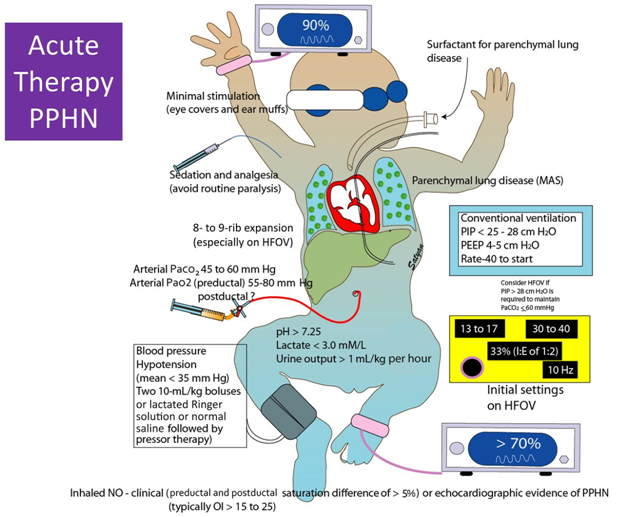
The treatment strategy for PPHN is aimed at maintaining adequate systemic blood pressure, decreasing pulmonary vascular resistance, ensuring oxygen release to tissues, and minimizing lesions induced by high levels of inspired oxygen and ventilator high pressure settings.
General management principles include the following:
- Continuous monitoring of oxygenation, blood pressure, and perfusion
- Maintaining a normal body temperature
- Correction of electrolytes/glucose abnormalities and metabolic acidosis
- Nutritional support
- Minimal stimulation/handling of the newborn
- Minimal use of invasive procedures (eg, suctioning) Medical therapy
- Inotropic support (eg, dopamine, dobutamine, milrinone). Although dopamine is frequently used as a first-line agent, other agents, such as dobutamine and milrinone, are helpful when myocardial contractility is poor.
- Surfactant administration: For premature and full-tem newborns with parenchymal lung disease
- Endotracheal intubation and mechanical ventilation: To maintain normal functional residual capacity by recruiting areas of atelectasis; to avoid overexpansion
- High-frequency ventilation: Used in newborns with underlying parenchymal lung disease and low lung volumes; therapy is best in centers with clinicians experienced in achieving/maintaining optimal lung distention
- Correction of acidosis and alkalosis
- Induced paralysis: Controversial; paralytic agents are typically reserved for newborns who cannot be treated with sedatives alone (Note: paralysis, especially with pancuronium, may promote atelectasis of dependent lung regions and promote ventilation-perfusion mismatch.)
- ECMO: Used when optimal ventilatory support fails to maintain acceptable oxygenation and perfusion [2, 3]
- Pulmonary vasodilators (eg, inhaled nitric oxide) and inhaled supplemental oxygen
- Vasodilators are potentially beneficial for chronic PPHN after the newborn period (eg, prostacyclin, phosphodiesterase inhibitors, endothelin receptor antagonists)
- Sedation and analgesia with opioids are often necessary to achieve adequate mechanical ventilation in patients with persistent pulmonary hypertension of the newborn (PPHN).
- The administration of surfactant may be helpful if parenchymal disease is present.
- Cardiac output is maintained with the use of inotropic agents and with judicious volume replacement.
- Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator that may decrease the need for invasive therapy, such as extracorporeal membrane oxygenation (ECMO).
- NO is a rapid and potent vasodilator that can be delivered through a ventilator because of its low molecular weight. Once in the bloodstream, it binds to hemoglobin, limiting its systemic vascular activity and increasing its selectivity for the pulmonary circulation.
- Treatment with iNO is indicated for newborns with an oxygen index (OI) of 25 or more. NO is an endothelially derived signalling molecule that relaxes vascular smooth muscle and that can be delivered to the lung by means of an inhalation device (INOvent; Ikaria, Clinton NJ). [36, 37]
- In 2 large randomized trials, NO reduced the need for ECMO support by approximately 40%. Although these trials led to the US Food and Drug Administration (FDA) approving iNO as a therapy for PPHN, iNO did not reduce mortality, the length of hospitalization, or the risk of neurodevelopmental impairment.
- A randomized study showed that beginning iNO at an earlier point in the disease course (for an OI of 15-25) did not decrease the incidence of ECMO and/or death or improve other patient outcomes, including the incidence of neurodevelopmental impairment.
- The use of iNO has not been demonstrated to reduce the need for ECMO in newborns with congenital diaphragmatic hernia. In these newborns, iNO should be used in non-ECMO centers to allow for acute stabilization, followed by immediate transfer to a center that can provide ECMO.
- Contraindications to iNO include congenital heart disease characterized by ductal dependent systemic blood flow (eg, interrupted aortic arch, critical aortic stenosis, hypoplastic left heart syndrome) and severe left ventricular dysfunction.
- Currently, the initial recommended concentration of iNO is 20 ppm. Higher concentrations are not more effective and are associated with a higher incidence of methemoglobinemia and formation of nitrogen dioxide. [38]
- In infants who respond, an improvement in oxygenation is evident within few minutes. Some studies have shown that concentrations of up to 5 ppm are effective in improving oxygenation. [39, 40] Lower concentrations (2 ppm) are not effective. [41]Once initiated, iNO should be gradually weaned to prevent rebound vasoconstriction.
- During iNO treatment, continuous monitoring of nitrogen dioxide and daily serum levels of methemoglobin should be obtained (methemoglobin levels should be kept at < 5%).
- Appropriate lung recruitment and expansion are essential to achieve the best response. If a newborn has severe parenchymal lung disease and PPHN, strategies such as high-frequency ventilation (HFV) may be required.
- In centers that do not have immediate availability of ECMO support, use of iNO must be approached with caution. Since iNO cannot be abruptly discontinued, transport with iNO is usually needed if a subsequent referral for ECMO is necessary. This capability should be determined in collaboration with the ECMO center before treatment is started. The use of iNO with HFV creates particular problems for transport, and this should be considered before these therapies are combined in a non-ECMO center. [42]
- Vasodilators potentially beneficial for persistent pulmonary hypertension beyond the newborn period
- Prostacyclin is a vascular endothelium-derived product of arachidonic acid metabolism with potent vasodilatory activity. It also has inhibitory effects on platelet aggregation, inflammation, and vascular smooth muscle proliferation. It has been used successfully in older patients with pulmonary hypertension but not commonly for PPHN. Treatment with epoprostenol in a randomized control trial has shown improvements over placebo in exercise capacity as assessed by the distance walked in 6 minutes, quality of life, pulmonary hemodynamics, and survival in idiopathic pulmonary hypertension. [43]
- Its use requires permanent vascular access as it has very short half life (~5 min), and any abrupt interruption in its delivery due to catheter dislodgment, blockage, or leak may result in potentially fatal rebound pulmonary hypertension. Its effect can wean overtime owing to the phenomenon of tachyphylaxis. [44] Common observed adverse effects are due to systemic vasodilation and include headache, dizziness, facial flushing, jaw pain, leg cramps, and gastrointestinal upset.
- Among the 11 isoforms of phosphodiesterases (PDEs), the most important are the PDE3 and PDE5, which degrade cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), respectively.
- Sildenafil, a PDE5 inhibitor was shown to selectively reduce pulmonary vascular resistance in both animal models and adult humans. It also has been reported to be successful in the treatment of infants with PPHN. [45]
- In a Cochrane meta-analysis with 37 newborns from centers that did not have access to NO and HFV, significant improvement in oxygenation was observed in the group receiving sildenafil. [46] This study noted that sildenafil may be a treatment option for PPHN.
- Additional studies are needed to assess the safety and efficacy of sildenafil compared with treatment with the more costly inhaled NO. [47]
- Abmen et al reported on the FDA's recent warning against using sildenafil for pediatric pulmonary hypotension in patients aged 1-17 years because of an apparent increase in mortality if used in high doses for long-term therapy. This warning, which is based on extremely limited data, indicates the need for further assessment of the efficacy and safety of sildenafil, especially with long-term treatment. [48]
- A more recent systematic review (2015) of off-label use of sildenafil in premature infants at risk for bronchopulmonary dysplasia (BPD) or BPD-associated pulmonary hypertension as well as term or near-term infants with pulmonary hypertension reported little evidence to support the use of sildenafil in term or near-term infants with PPHN in areas where NO is available. [49] The investigators also noted the need for more data sildenafil dosing, safety, and efficacy in premature, term, and near-term infants with pullmonary hypertension. [49]
- The endothelins (ETs) comprise a family of three 21-amino acid peptides, ET-1, ET-2, and ET-3. Of these, only ET-1 plays an important role in the regulation of vascular tone. ET-1 is a very potent vasoconstrictor and smooth muscle mutagen produced primarily by vascular endothelial cells. [50, 51]
- The development of the endothelin receptor antagonists represents an important milestone in the therapeutic approach for pulmonary hypertension.
- Bosentan is the first orally active treatment to show efficacy in a randomized trial of pulmonary arterial hypertension. Randomized controlled trials and systematic reviews in adults have shown that bosentan improves the outcomes of patients with pulmonary hypertension. [52, 53] There are 2 main concerns: the potential for serious hepatic injury and teratogenic effects. Monthly monitoring of liver function tests for the duration of treatment is mandatory, as approximately 10% of patients receiving bosentan show an increase in liver transaminase levels of 3-fold or greater. [54] To avoid the risk of major birth defects in women exposed to endothelin antagonists, effective contraception must be practiced and monthly pregnancy testing is required.
- Studies have shown that Rho A/Rho kinase activation causes pulmonary vasoconstriction and promotes pathogenic vascular remodeling. Previous investigators have demonstrated that inhibition of the activity of Rho A/Rho kinases using the Rho kinase inhibitor fasudil has beneficial effects on the pulmonary vasculature on different animal models of pulmonary hypertension. [55]
PPHN treatment may consist of the following:
 Pharmacotherapy
Pharmacotherapy
Inhaled nitric oxide
Magnesium sulfate promotes vasodilatation by antagonizing the entry of calcium ions into the smooth muscle cells. [56] However, its pulmonary vasodilator properties have not been studied in the adult or pediatric population.
Combination therapy offers a greater option to enhance pulmonary vasodilatation compared with monotherapy. For example, NO can work synergistically with PDE-5 inhibitors to increase cGMP levels; prostacyclin (PGI2) (which enhances c-AMP) can work synergistically with NO (which enhances c-GMP); endothelin receptors antagonists can work synergistically with NO.
REFERENCES:
1. Cabral JE, Belik J. Persistent pulmonary hypertension of the newborn: recent advances in pathophysiology and treatment. J Pediatr (Rio J). 2013 May-Jun. 89(3):226-42. [Medline].
2. Bahrami KR, Van Meurs KP. ECMO for neonatal respiratory failure. Semin Perinatol. 2005 Feb. 29(1):15-23. [Medline].
3. Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol. 2005 Feb. 29(1):8-14. [Medline].
4. Cassin S, Dawes GS, Mott JC, Ross BB, Strang LB. The vascular resistance of the foetal and newly ventilated lung of the lamb. J Physiol. 1964 May. 171:61-79. [Medline]. [Full Text].
5. Dawes GS, Mott JC, Widdicombe JG, Wyatt DG. Changes in the lungs of the new-born lamb. J Physiol. 1953 Jul. 121(1):141-62. [Medline].
6. Berti A, Janes A, Furlan R, Macagno F. High prevalence of minor neurologic deficits in a long-term neurodevelopmental follow-up of children with severe persistent pulmonary hypertension of the newborn: a cohort study. Ital J Pediatr. 2010 Jun 13. 36:45. [Medline]. [Full Text].
7. Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol. 2009. 191:309-39. [Medline].
8. Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol. 2009. (191):277-308. [Medline].
9. Jaillard S, Larrue B, Deruelle P, et al. Effects of phosphodiesterase 5 inhibitor on pulmonary vascular reactivity in the fetal lamb. Ann Thorac Surg. 2006 Mar. 81(3):935-42. [Medline].
10. Villamor E, Le Cras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol. 1997 May. 272(5 Pt 1):L1013-20. [Medline].
11. Hanson KA, Ziegler JW, Rybalkin SD, Miller JW, Abman SH, Clarke WR. Chronic pulmonary hypertension increases fetal lung cGMP phosphodiesterase activity. Am J Physiol. 1998 Nov. 275(5 Pt 1):L931-41. [Medline].
12. Pearson DL, Dawling S, Walsh WF, et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001 Jun 14. 344(24):1832-8. [Medline].
13. Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Mitchell AA. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics. 2007 Aug. 120(2):e272-82. [Medline].
14. Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. 2006 Feb. 30(1):34-43. [Medline].
15. US food and drug administration. FDA Drug Safety Communication: Selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. Available at http://www.fda.gov/drugs/drugsafety/ucm283375.htm. Accessed: December 14, 2011.
16. Teng RJ, Wu TJ. Persistent pulmonary hypertension of the newborn. J Formos Med Assoc. 2013 Apr. 112(4):177-84. [Medline].
17. Atkinson JB, Ford EG, Kitagawa H, Lally KP, Humphries B. Persistent pulmonary hypertension complicating cystic adenomatoid malformation in neonates. J Pediatr Surg. 1992 Jan. 27(1):54-6. [Medline].
18. Robin H Steinhorn, MD and Kathryn N Farrow, MD, PhD. Pulmonary hypertension in the neonate. NeoReviews. January 1, 2007. 8:e14 -e21. [Full Text].
19. Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000 Jan. 105(1 Pt 1):14-20. [Medline].
20. Steinhorn RH, Kinsella JP, Pierce C, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009 Dec. 155(6):841-847.e1. [Medline].
21. Yoder BA, Kirsch EA, Barth WH, Gordon MC. Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol. 2002 May. 99(5 Pt 1):731-9. [Medline].
22. Yeh TF. Core concepts: Meconium aspiration syndrome: Pathogenesis and current management. NeoReviews. 2010 Sep. 11(9):e503-12. [Full Text].
23. Weijerman ME, van Furth AM, van der Mooren MD, et al. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr. 2010 Oct. 169(10):1195-9. [Medline].
24. Sallaam S, Natarajan G, Aggarwal S. Persistent pulmonary hypertension of the newborn with D-transposition of the great arteries: management and prognosis. Congenit Heart Dis. 2015 Nov 11. [Medline].
25. Silva DM, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2015 Dec 1. 309 (11):L1239-72. [Medline].
26. Malowitz JR, Forsha DE, Smith PB, Cotten CM, Barker PC, Tatum GH. Right ventricular echocardiographic indices predict poor outcomes in infants with persistent pulmonary hypertension of the newborn. Eur Heart J Cardiovasc Imaging. 2015 Nov. 16 (11):1224-31. [Medline].
27. Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015 Nov 24. 132 (21):2037-99. [Medline].
28. Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Survanta in Term Infants Study Group. J Pediatr. 1998 Jan. 132(1):40-7. [Medline].
29. Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996 Jan. 97(1):48-52. [Medline].
30. Brown KL, Sriram S, Ridout D, et al. Extracorporeal membrane oxygenation and term neonatal respiratory failure deaths in the United Kingdom compared with the United States: 1999 to 2005. Pediatr Crit Care Med. 2010 Jan. 11(1):60-5. [Medline].
31. Hintz SR, Suttner DM, Sheehan AM, Rhine WD, Van Meurs KP. Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization. Pediatrics. 2000 Dec. 106(6):1339-43. [Medline].
32. Christou H, Van Marter LJ, Wessel DL, et al. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Crit Care Med. 2000 Nov. 28(11):3722-7. [Medline].
33. Lazar DA, Cass DL, Olutoye OO, et al. The use of ECMO for persistent pulmonary hypertension of the newborn: a decade of experience. J Surg Res. 2012 Oct. 177(2):263-7. [Medline].
34. Laffey JG, Engelberts D, Kavanagh BP. Injurious effects of hypocapnic alkalosis in the isolated lung. Am J Respir Crit Care Med. 2000 Aug. 162(2 Pt 1):399-405. [Medline].
35. Wung JT, James LS, Kilchevsky E, James E. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985 Oct. 76(4):488-94. [Medline].
36. Konduri GG, Solimano A, Sokol GM, et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004 Mar. 113(3 Pt 1):559-64. [Medline].
37. Steinhorn RH. Nitric oxide and beyond: new insights and therapies for pulmonary hypertension. J Perinatol. 2008 Dec. 28 Suppl 3:S67-71. [Medline].
38. American Academy of Pediatrics. Committee on Fetus and Newborn. Use of inhaled nitric oxide. Pediatrics. 2000 Aug. 106(2 Pt 1):344-5. [Medline].
39. Kinsella JP, Walsh WF, Bose CL, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999 Sep 25. 354(9184):1061-5. [Medline].
40. Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. The I-NO/PPHN Study Group. Pediatrics. 1998 Mar. 101(3 Pt 1):325-34. [Medline].
41. Clark RH, Kueser TJ, Walker MW, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000 Feb 17. 342(7):469-74. [Medline].
42. Pawlik TD, Porta NF, Steinhorn RH, Ogata E, deRegnier RA. Medical and financial impact of a neonatal extracorporeal membrane oxygenation referral center in the nitric oxide era. Pediatrics. 2009 Jan. 123(1):e17-24. [Medline].
43. Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996 Feb 1. 334(5):296-301. [Medline].
44. Barst RJ, Rubin LJ, McGoon MD, Caldwell EJ, Long WA, Levy PS. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med. 1994 Sep 15. 121(6):409-15. [Medline].
45. Ahsman MJ, Witjes BC, Wildschut ED, et al. Sildenafil exposure in neonates with pulmonary hypertension after administration via a nasogastric tube. Arch Dis Child Fetal Neonatal Ed. 2010 Mar. 95(2):F109-14. [Medline].
46. Shah PS, Ohlsson A. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2007 Jul 18. CD005494. [Medline].
47. Shah PS, Ohlsson A. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2011 Aug 10. CD005494. [Medline].
48. Abman SH, Kinsella JP, Rosenzweig EB, et al. Implications of the U.S. Food and Drug Administration warning against the use of sildenafil for the treatment of pediatric pulmonary hypertension. Am J Respir Crit Care Med. 2013 Mar 15. 187(6):572-5. [Medline].
49. Perez KM, Laughon M. Sildenafil in term and premature infants: a systematic review. Clin Ther. 2015 Nov 1. 37 (11):2598-2607.e1. [Medline].
50. Dakshinamurti S. Pathophysiologic mechanisms of persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2005 Jun. 39(6):492-503. [Medline].
51. Abman SH. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu Rev Med. 2009. 60:13-23. [Medline].
52. Galie N, Rubin Lj, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008 Jun 21. 371(9630):2093-100. [Medline].
53. Liu C, Chen J, Gao Y, Deng B, Liu K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2009 Jul 8. CD004434. [Medline].
54. Humbert M, Segal ES, Kiely DG, Carlsen J, Schwierin B, Hoeper MM. Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 2007 Aug. 30(2):338-44. [Medline].
55. Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol. 2008 Oct. 155(4):444-54. [Medline]. [Full Text].
56. Iseri LT, French JH. Magnesium: nature's physiologic calcium blocker. Am Heart J. 1984 Jul. 108(1):188-93. [Medline].
Source: Salaam Sallaam in Medscape.
Available from: https://emedicine.medscape.com/article/898437-overview

