Neonatology
Use of Lung Ultrasound to Improve Timeliness of Surfactant Replacement in RDS: Are we Ready?
The management of respiratory distress syndrome (RDS) is generally based on the clinical presentation and the increasing fraction of inspired oxygen (FiO2) requirement, which guides clinicians to consider administering surfactant. However, the dilemma regarding the ideal time to administer surfactant still exists. Early use of surfactant (within 2 hours of postnatal life) in RDS will decrease pneumothorax and bronchopulmonary dysplasia, and improve survival.1, 2, 3 The identification of an infant who requires surfactant is usually based on the infant's requirement of supplemental O2.3 In the current era, noninvasive ventilation in the form of continuous positive airway pressure (CPAP) at birth has become popular to avoid intubations and invasive mechanical ventilation. In such situations, it has also become challenging to decide when infants should be intubated based on increasing FiO2 while the infant is on CPAP, in the absence of additional objective criteria.
The recent European consensus guidelines on the management of RDS-2019 Update suggest that infants with RDS should be given rescue surfactant early when infant is requiring a FiO2 >0.3 on CPAP of at least 6 cm H2O.3The guideline also suggests that second and third doses of surfactant should be given if there is persistent increasing FiO2 and persistence of RDS-like picture.3 However, these guidelines have not mentioned an exact cut-off value of FiO2 for administration of the second dose of surfactant.
Studies have shown that use of FiO2 as a diagnostic criterion for deciding about administration of surfactant has a lower accuracy than lung ultrasound.4, 5, 6 This modality was first used in the neonatal intensive care unit (NICU) almost a decade ago and is rapidly gaining popularity in modern NICUs.7, 8 However, its role in decision making about the administration of surfactant in premature infants has not been explored extensively due to multiple factors including availability of ultrasound machine in the NICUs, training of personnel, and lack of evidence.
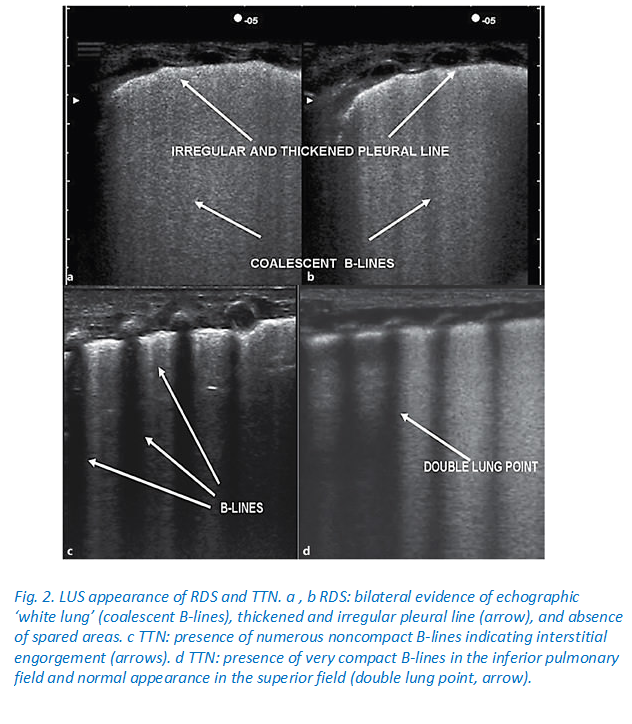
Copetti et al evaluated the role of lung ultrasound in 40 preterm infants (gestational age of 27.2 ± 2.7 weeks) with radiologic findings of RDS and found that lung ultrasound was a reliable screening tool for diagnosis of RDS and administration of surfactant.8 However, this study was not blinded, and, therefore, the risk of bias existed.8Studies have developed a lung ultrasound score and used it to evaluate lung aeration and prediction of administration of surfactant when neonates were on CPAP for RDS.5, 6 A study by Brat et al showed that the lung ultrasound score is a better predictor of the need for surfactant in preterm infants with gestational age of <34 weeks (area under the curve: 0.93; 95% CI 0.86-0.99, P < .01) than late preterm and term neonates (area under the curve: 0.71; 95% CI 0.54-0.90, P < .02).5 This study included a total 130 neonates (gestational age of <34 weeks, n = 65; neonates ≥34 weeks, n = 65).5 In another study, lung ultrasound was predictive of the need for surfactant replacement in premature infants born ≤30 weeks with RDS and treated with CPAP.6 Lung ultrasound performed just before surfactant administration accurately predicted the need for the first surfactant dose (area under the curve: 0.94; 95% CI 0.90-0.98, P < .0001) and also the need for the second dose of surfactant (area under the curve: 0.80; 95% CI 0.72-0.89, P < .0001) for premature infants ≤30 weeks of gestational age.6 The limitations of other lung ultrasound studies included not performing ultrasound just before surfactant administration, no studies in <26 weeks of gestational age infants, lower predictive accuracy in late preterm and term infants, as well as for the second dose of surfactant administration.5, 7, 9 None of the studies have assessed the sensitivity and specificity of the composite of lung ultrasound score in addition to the requirement of FiO2 vs FiO2 requirement only to predict the need of surfactant administration in extremely premature infants with RDS.5, 6, 7, 8, 9
In a recent pilot study published in this volume of The Journal, Raschetti et al address the above question as a quality improvement project using either a lung ultrasound score or FiO2 thresholds compared with FiO2thresholds only, to guide surfactant replacement therapy.10 This study demonstrated that echography-guided surfactant therapy (ESTHER) improves the timeliness of surfactant administration in neonates born ≤32 weeks of gestation with RDS.10 In this study, in the first 6 months of the “Plan-Do-Study-Act” cycle, surfactant was administered in the first 72 hours of life if the FiO2 increased above 0.3 for infants ≤286/7 weeks and above 0.4 for infants ≥290/7 weeks of gestation.10 In the second half of the study period, ESTHER criteria were used for surfactant administration including exceeding FiO2 limits as above or the lung ultrasound score higher than 8, whichever occurred first.10 It is not explicitly stated if this was irrespective of the type of respiratory support, though it is implied that the infant would be on CPAP in all instances. It is also not clear if the lung ultrasound was performed at specific times after the birth of the baby, which would obviously impact on the lung ultrasound score.
The proportion of neonates who received surfactant within the first 3 hours of life was significantly increased (71%-90%; P < .001) and the maximal FiO2 reached was reduced before surfactant replacement therapy (33%-40%).10 The authors did not find any difference in the overall need for surfactant.10 In addition to this, the Raschetti et al study also mentioned that there was a reduction in the need for invasive ventilation and increased ventilator-free days in the ESTHER group.10
Using the “before-after” approach (similar to using “historical controls”) and the fact that the lung ultrasound is obviously unblinded does run the risk of potential bias. Obviously, generalization of the results would be difficult because lung ultrasound is a learned skill, and this skill set may not be transferable as easily as practiced in the study NICU. Given that this was a quality improvement project, it was not powered to measure robust clinical outcomes for the infants. The sample size was also relatively small. We agree with the authors10 that there is no requirement of extra cost to introduce ESTHER criteria in NICUs, if there is already a point of care (POC) ultrasound machine available in the NICUs. However, time will be needed to provide adequate training for lung ultrasound.
The use of ultrasound as a POC tool is increasingly being used in NICUs worldwide by neonatologists for a variety of purposes.11, 12, 13 The use of POC lung ultrasound is becoming a practical option in modern NICUs for detection and/or management of various pulmonary diseases.14, 15 The lung ultrasound has some benefits including lower exposure to radiation, easy to perform at bedside, can be repeated several times a day, and has a low cost. Lung ultrasound cannot replace chest radiograms for diagnosing of secondary complications of RDS, especially air leak syndromes, though pneumothorax can be detected by lung ultrasound.16 The results of the lung ultrasound are influenced by respiratory support, gestational age, fluid intake, and pre-existing lung conditions. The POC ultrasound, including lung ultrasound, has a steep learning curve. and this modality does require acquisition of skills with training and practice by medical personnel in the NICU to gain experience to be able to use it effortlessly in modern NICUs. The availability of a lung ultrasound machine with an appropriate high resolution and a microlinear probe is a necessity for the ideal imaging of lungs. In the absence of enough evidence from multiple studies using lung ultrasound imaging in extremely premature infants, especially <26 weeks of gestational age, the use of lung ultrasound is unlikely to become a part of the standard of care of premature infants in modern NICUs. There is a need for large multicenter studies to determine the role of lung ultrasound for the prediction of early administration of surfactant to determine the appropriate neonatal outcomes.
References
- Pediatrics. 2014; 133: 156–163
- Cochrane Database Syst Rev. 2012; 11: CD001456
- Neonatology. 2019;115: 432–451
- Qual Saf Health Care. 2010; 19: e23
- JAMA Pediatr. 2015; 169: e151797
- Pediatrics. 2018; 142: e20180463
- Neonatology. 2007; 91: 203–209
- Neonatology. 2008; 94: 52–59
- Neonatology. 2014; 106: 87–93
- J Pediatr. 2019; 212: 137–143
- J Ultrasound. 2019; 22: 201–206
- J Wound Ostomy Continence Nurs. 2018; 45: 503–509
- J Ultrasound Med. 2016; 35: 1579–1591
- Liu J, et al. (in press) J Vis Exp. 2019;
- Liu, J., Xia, R.M., Ren, X.L., and Li, J.J. (in press)J Matern Fetal Neonatal Med. 2019;
- Am J Emerg Med. 2017; 35: 1298–1302
(Available from: https://www.jpeds.com/article/S0022-3476(19)30674-2/pdf)

