Equipment Review
'NEO THERM'- an indigenous, servo-controlled, whole-body cooling system
"There is now unequivocal and high-quality evidence from multiple large RCTs that neonates with moderate to severe hypoxic-ischemic encephalopathy (HIE) following a perinatal event benefit from hypothermia under tightly controlled conditions"
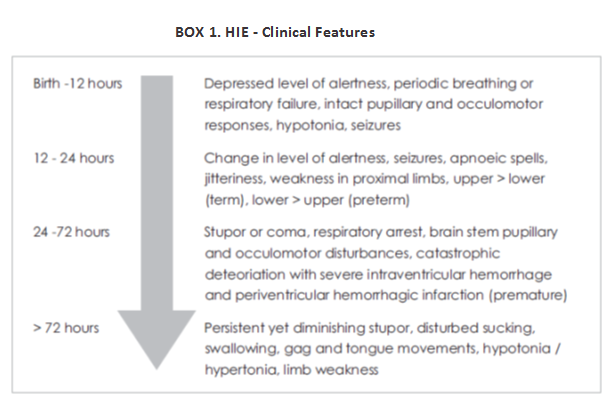
An estimated 4 million babies die every year in the neonatal period. Ninety nine percentage of these deaths are in low to middle income countries. It is a sad fact that most of the neonatal deaths due to asphyxia (99%) occur in developing countries. India contributes to one-fifth of global live births and more than a quarter of neonatal deaths. HIE occurs in 1.5 per 1000 full term births. While 15 - 20% of neonates with HIE die early, 25% will survive with disabilities. Moreover HIE is a major problem at all levels - individual, family and society, contributing to 15-28% of children with cerebral palsy and 25% of all children with developmental delay. Despite significant advances in perinatal care, cerebral palsy among term infants continues to occur.
A recent systematic analysis has shown that low technology device in tertiary level settings has improved survival and neurological outcome in HIE babies with moderate to severe encephalopathy.

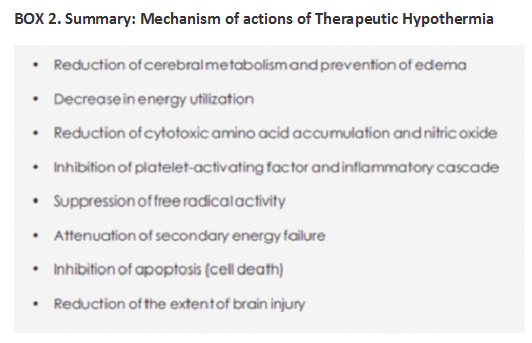
Therapeutic Hypothermia (TH) has been proven to be effective in reducing morbidity associated with HIE and has become the standard of care for HIE in developed countries. However, in underdeveloped and transitional countries where the problem is more common, therapeutic cooling is still in the nascent phase. As of today, the only modality for treatment and a means to decrease long term morbidity in babies with perinatal asphyxia is the initiation of therapeutic hypothermia within the first 6 hours of life.

Early application of TH preferably within 6 hours i.e. before the onset of the secondary phase of energy failure is likely to be effective and improve neurodevelopmental outcome. Usually it is continued for a period of 72 hours for better neuro protection. There is also evidence that therapeutic hypothermia limits myocardial and renal injury in term infants with HIE.
WHICH NEONATES TO COOL?
Neonates can be considered for cooling if the following criteria are met (NICHD criteria):
1.Gestational age ≥ 36 weeks
2. Less than 6 hours old
3. A pH of 7.0 or less or a base deficit of 16 mmol per liter or more in a sample of umbilical cord blood or any blood during the first hour after birth.
If the pH is between 7.01 and 7.15, a base deficit is between 10 and 15.9 mmol per liter,
If a blood gas is not available, additional criteria are required. These include an acute perinatal event (e.g., late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, hemorrhage, or cardiorespiratory arrest) and either a 10-minute Apgar score of 5 or less or assisted ventilation initiated at birth and continued for at least 10 minutes.
4. Moderate to severe encephalopathy on clinical examination.
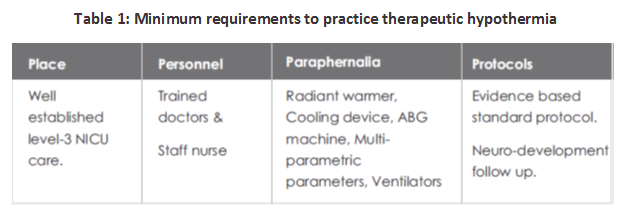
PREREQUISITES TO PRACTICE THERAPEUTIC HYPOTHERMIA
The minimum prerequisites to start the practice of therapeutic hypothermia have been depicted in Table.1:
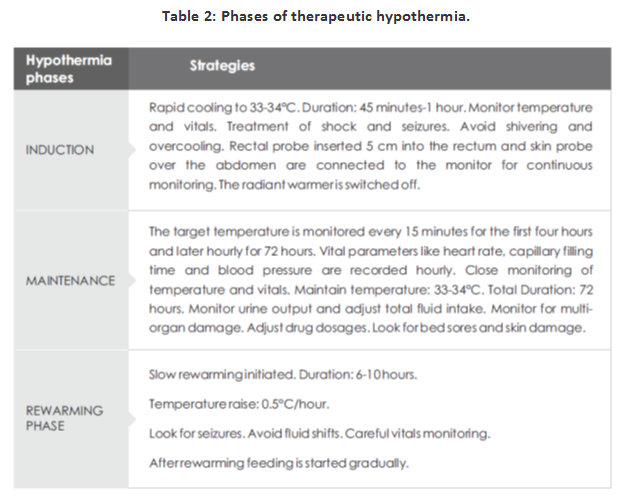
DIFFERENT PHASES OF COOLING THERAPY
The different phases of therapeutic hypothermia are depicted in the Table 2.
MONITORING OF NEONATES DURING THERAPEUTIC HYPOTHERMIA
As cooling is used more widely and has been newly introduced in neonatal units, continued surveillance of its use in clinical practice is mandatory. The initial management of infants with HIE following admission to the neonatal unit consists of standard neonatal intensive care measures, continuous core temperature monitoring using a rectal probe and initiation of therapeutic hypothermia. Monitoring of such infants is critical to the neurological outcome.
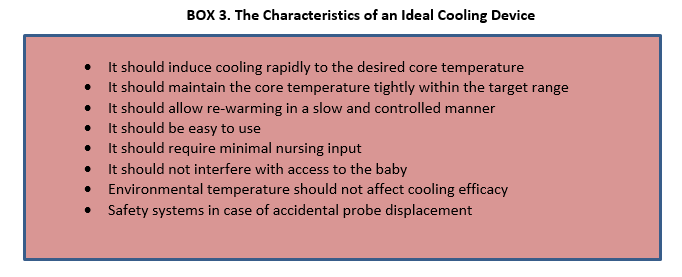
COOLING DEVICES
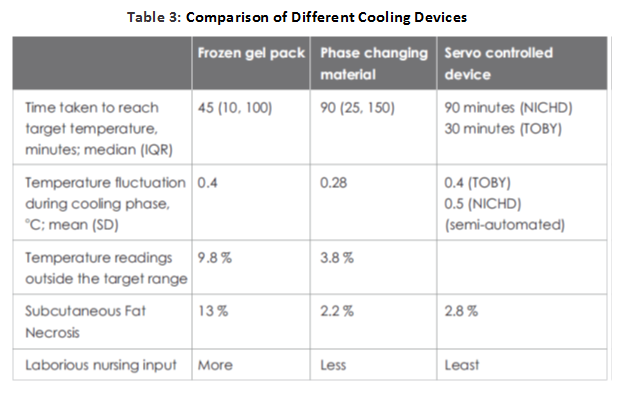
Various low technologies methods like passive cooling, ice gel pack, cooling fan, etc.; have been tried to achieve therapeutic hypothermia because of the high cost and unavailability of servo-controlled equipment. There have been concerns of overcooling, fluctuations in temperature, cold injury like subcutaneous fat necrosis, increased shivering and need of more nursing input.
While most western centers currently prefer systemic hypothermia delivered using servo-controlled mattresses, these devices are expensive. Passive cooling [by fans, ice/gel packs and phase-changing materials (PCMs)] is a very practical and inexpensive option, especially for transporting outborn infants who may not arrive in an NICU within the 6-hour window for initiating hypothermia. But some infants do not cool enough with passive cooling, while others may overshoot the target temperature.

The use of phase changing material (PCM) (MiraCradle-TM by M/s VNG Medical Innovation System) (Figure 2 ) for cooling is increasingly popular in India because of the availability of a relatively inexpensive device. This has been studied as an alternative to servo-controlled cooling and appears to perform reasonably well, with one study reporting that the target temperature was maintained during 96.2% of the cooling phase.
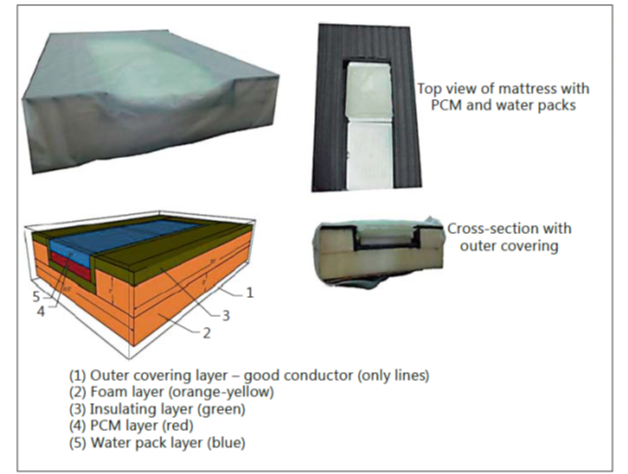
Figure 2. Construction of the PCM bed. The bed consists of an outer covering made of nylon (1), a hollowed-out foam layer (2) lined with an insulating material like extruded polystyrene (3), PCM blocks kept in the hollowed-out area (4) and a water mattress above the PCM blocks and below the outer covering (5).
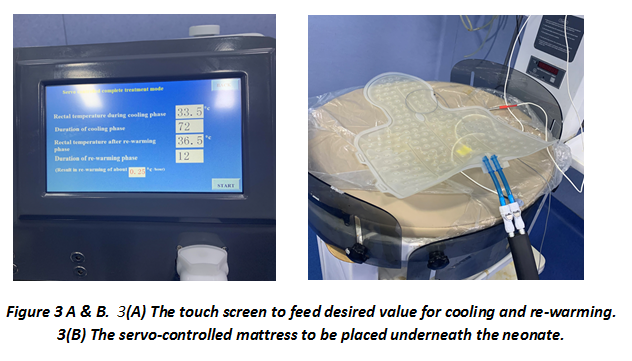
NEO THERM-TM An indigenous, servo-controlled, whole-body cooling system for birth asphyxia
Recently, a new low-cost, servo-controlled mattress-based device, NEO THERM-TM by the M/S VNG Medical Innovation System is introduced in the market (Figures 1 & 3). It is an upgrade model of their earlier MiraCradle-TM unit with servo-controlled cooling and rewarming facility. This consists of a mattress that is placed below the baby or can also be wrapped around baby's trunk. The device is fully automated and requires no further input after initiation. The baby's temperature is cooled to 33°50C within 30 minutes. This temperature is maintained for 72 hours and a fully automated rewarming process starts. The rewarming occurs to 37°C at 0.5°C/hour (requiring around 12 hours for rewarming). The monitor displays rectal and skin temperature. Default temperature settings on the machine can be altered according to individual needs.
DURATION OF COOLING
The duration of hypothermia in most trials was 72 hours. One trial cooled infants for 48 hours and another for 48 to 72 hours depending on the neurological status of the infant. Cooling at 33°50C for 72 hours in term infants with moderate to severe HIE is now considered safe and beneficial, and current evidence therefore suggests that cooling for a duration longer than 72 hours is not beneficial.
DEPTH OF COOLING
Deeper cooling did not appear to be beneficial. There is currently no evidence to cool below the commonly used target temperature of 33°50C (range of 33°C-34°C).
REWARMING
There is concern about decrease in systemic blood pressure during rewarming. Rebound seizures can occur during rewarming. Rewarming has also been reported to affect the EEG background. However, it is not clear if another regime for rewarming will be better than the ones used in the RCTs. With current evidence, it appears prudent to rewarm slowly, at a rate of no more than 0°50C per hour for over 12 hours.
COOLING PRETERM INFANTS
Most RCTs have been done in term and late preterm infants, with a gestational age of 35 weeks or higher. Most enrolled infants in these trials were born at term. Initial reports of cooling preterm infants indicate that caution is warranted. Trials are undergoing to assess safety and utility of cooling preterm infants of 33-35 weeks gestation. Until more evidence is generated by this and other trials, hypothermia should not be offered to infants below 35 weeks of gestation.
MONITORING OF NEONATES DURING THERAPEUTIC HYPOTHERMIA
As cooling is used more widely and has been newly introduced in neonatal units, continued surveillance of its use in clinical practice is mandatory. The initial management of infants with HIE following admission to the neonatal unit consists of standard neonatal intensive care measures, continuous core temperature monitoring using a rectal probe and initiation of therapeutic hypothermia. Monitoring of such infants is critical to the neurological outcome.
Temperature monitoring
Both the core temperature and skin temperature are monitored continuously. The core temperature is usually monitored by rectal or oesophageal probe. The rectal probe should be inserted 3-6 cm and secured to the thigh. The temperature is recorded every 15 minutes till a core temperature of 33°5°C is achieved and thereafter every hour till rewarming is completed. The core temperature should be maintained between 33°5°C to 34°C. The fluctuation of temperature during the maintenance phase should be very minimal as it can adversely affect the neurological outcome.
Cardiorespiratory care
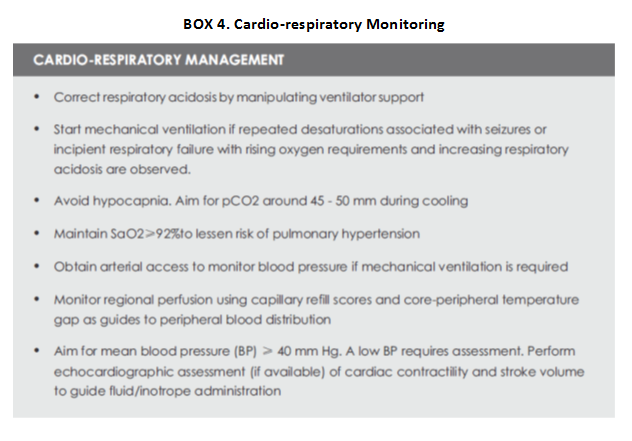
Hypothermia is consistently associated with sinus bradycardia as it slows the atrial pacemaker and intracardiac conduction. The heart rate can be even lower than 100. Heart rate drops by nearly 14 beats per one degree drop in temperature. HR up to 70 can be tolerated as long as it is normal sinus rhythm and normal SpO2 and BP are observed (Box 4). Sinus bradycardia usually does not need intervention. Close monitoring of BP is very essential to identify shock since CRT will be not a reliable indicator of hypo perfusion in babies receiving cooling therapy. All infants should be monitored closely with invasive blood pressure monitoring (if possible) and treated with inotropes accordingly.
Mechanical ventilation
Ventilation may not be needed for all neonates during cooling, however, it is important to maintain normal pCO2 , pO2 , pH and respiratory effort. Decreasing body temperature lowers metabolic rate by about 5-8% per °C. Furthermore, partial pressure of blood gases and pH are also affected because of altered gas solubility during hypothermia. With each degree Celsius decrease in core temperature, pH increases by 0.015, pCO2 and pO2 decrease by 4% and 7% respectively (Box 4). Excessively low pCO during therapeutic hypothermia may result in altered cerebral blood flow auto regulation, and reduced cerebral perfusion, and may lower the seizure threshold.
Skin monitoring
Poor skin perfusion occurs during cooling. The skin has to be inspected periodically and the back to be inspected at least 12 hourly. The position has to be changed 6 to 12 hourly from flat to slightly tilted in the supine position. Cyanosis of the hands and feet is common and usually transient.
Fluid balance
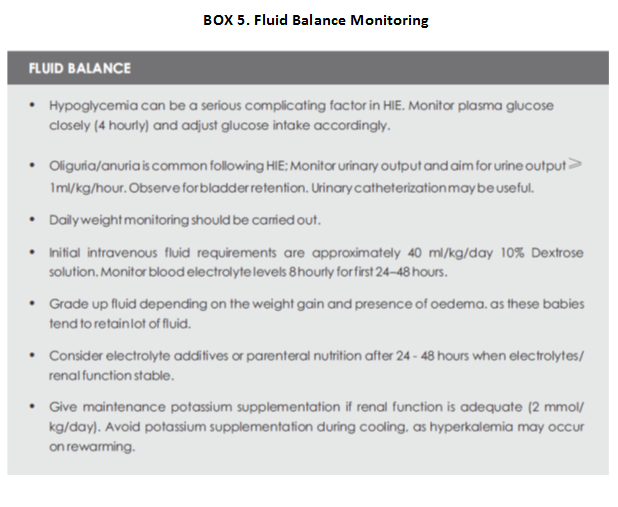
Disturbances in electrolytes and glucose homeostasis are common in infants with HIE, including those receiving therapeutic hypothermia. Fluid balance is essential in cooling neonates as the metabolic rate is low, and they may not need large volume of fluids (Box 5).
Fluid boluses should be avoided as it can lead to exacerbation of oedema which is due to capillary leak. The cooled babies may need more volume during rewarming phase due to redistribution of fluid into the tissues and increased diuresis. It is recommended that serum electrolytes and plasma glucose should be kept within the normal range during hypothermia treatment. Hypocalcemia, hypomagnesemia, and hypoglycemia are common in asphyxiated newborn infants.
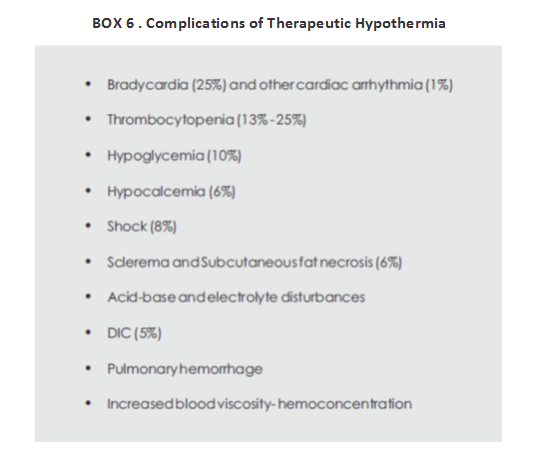
COMPLICATIONS OF THERAPEUTIC HYPOTHERMIA
Though therapeutic hypothermia is generally safe, it is important to cool neonates using strict protocols and often at centers with considerable experience with cooling.
Excessive cooling can cause cardiovascular instability and re-emergence of seizures. Some of the problems associated with cold injury syndrome include increased mortality; development of sclerema, skin erythema, and acrocyanosis; pulmonary hemorrhage; renal failure; increased blood viscosity and DIC; hypoglycemia; acid base and electrolyte disturbances; increased risk of infections (secondary to decreased leukocyte mobility and phagocytosis); significant cardiovascular disturbances including sudden cardiac arrest and ventricular tachyarrhythmia. Sinus bradycardia and thrombocytopenia have been reported to be the only significant adverse effects of hypothermia (Box 6).