Vaccinology Update
Who is at risk of PCV-13 vaccine failure?
PCV13 vaccine failure is more frequent among older children with underlying comorbidity, and among those who present with pneumococcal pneumonia.
Despite high vaccine coverage rates in children and efficacy of pneumococcal conjugate vaccines, invasive pneumococcal disease (IPD) episodes due to serotypes included in the vaccine following completion of the recommended course of immunisation (i.e. vaccine failure) have been reported.
The researchers used data gathered from a population-based enhanced passive surveillance for IPD in children under 18 years of age in Massachusetts and an ensemble model composed of three machine learning algorithms to predict probability of 13-valent pneumococcal conjugated vaccine (PCV13) failure and to evaluate potential associated features including age, underlying comorbidity, clinical presentation, and vaccine schedule. Vaccine failure was defined as diagnosis of IPD due to vaccine serotype (VST), in a child who received age recommended doses recommended by Advisory Committee of Immunization Practices.
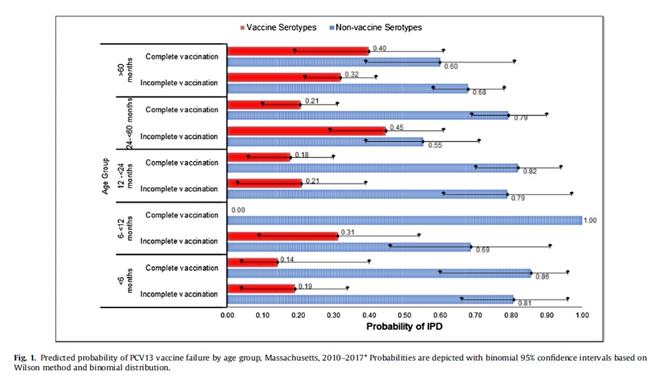
Results: During the 7-year study period, between April 01, 2010 and March 31, 2017, they identified 296 IPD cases. There were 107 (36%) IPD cases caused by VST, mostly serotype 19A (49, 17%), 7F (21, 7%), and 3 (18, 6%). Thirty-seven (34%) were in children who were completely vaccinated representing 13% of all IPD cases. Vaccine failure was more likely among children older than 60 months (predicted probability 0.40, observed prevalence 0.37, model prediction accuracy 79%), children presenting with pneumonia (predicted probability 0.27, observed prevalence 0.31, model accuracy 77%), and children with underlying comorbidity (predicted probability 0.24, observed prevalence 0.23, model accuracy 96%). Vaccine failure probability for those >60 months of age and had an underlying risk factor was 45% (observed prevalence 0.33, model accuracy 82%). The likelihood of vaccine failure was lowest among children who had completed 3 primary doses plus one booster dose PCV13 (predicted probability 0.14, observed prevalence 0.14, model prediction accuracy 100%).
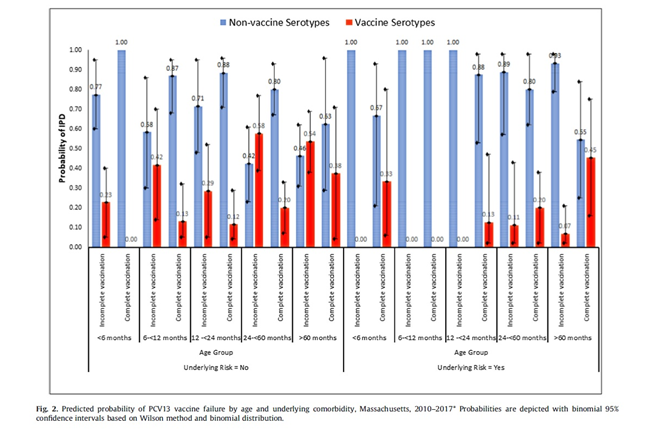
There were 37 cases in children who were completely vaccinated yet developed IPD due to serotypes included in PCV13 (vaccine failure 12.5%). Vaccine failure was more likely among older children (i.e. >60 months of age) who were completely vaccinated with PCV13 yet developed IPD and those presenting with pneumonia. If an older child (i.e. >60 months of age) with an underlying comorbidity presented with IPD, it was more likely to be a vaccine failure compared to children without any underlying comorbidity. The main serotypes associated with vaccine failure were 19A, 7F, 3.
Children with certain underlying comorbidity are at higher risk for developing IPD irrespective of vaccination status (i.e. sickle cell disease, prematurity, immunodeficiency, etc.). In a recent systemic review, almost one third of the PCV7 or PCV10 vaccine failure cases were reported to have at least one underlying comorbidity. This phenomenon also was reported with Hib vaccine failures where half of the cases had an underlying condition such as malignancy, prematurity, or Down syndrome.
Majority of the vaccine failures in this study were due to serotypes 19A, 7F and 3. These serotypes are still in circulation in community and contributed to approximately 10% of the colonizing
serotypes isolated from children <7 years of age in Massachusetts following implementation of PCV13. Although anticapsular polysaccharide antibody concentration of 0.35 mg/mL has been accepted as the correlate of protection, serotype specific differences have been reported. The anti-capsular antibody threshold of 0.35 lg/ml or more, or OPA 8 or more are accepted correlates of protection against IPD. It is possible that higher antibody concentrations are required to prevent hospitalized and outpatient pneumonia.
The potential for higher likelihood of vaccine failure for pneumonia cases is consistent with several reports from different settings and worth further exploring.
The above study contributes to the existing evidence that age appropriate immunization with PCV13, significantly decreases the estimated probability of VST IPD. However, different schedules are in use for different countries. In the US, routine 3 + 1 schedule was shown to provide 86% effectiveness against serotypes included in the vaccine. In England, vaccine effectiveness for 2 + 1 schedule was reported as 75% for PCV13 serotypes. In this study addition of a booster dose was associated with a less likelihood of vaccine failure for all children, but 2 doses or 3 primary doses didn’t change the predicted vaccine failure rates. However, for children who had at least one underlying comorbidity, receiving 3 primary doses or 3 primary doses plus a booster dose reduced the probability of vaccine failure. All IPD cases among these children were due to NVST if they had received at least 3 doses of PCV13.
Conclusion: PCV13 vaccine failure is more frequent among older children with underlying comorbidity, and among those who present with pneumococcal pneumonia. Our study provides a preliminary framework to predict the patterns of vaccine failures and may contribute to decision-making processes to optimize PCV immunization schedules.
This study provides a preliminary framework to predict the patterns of vaccine failure IPD cases which may be used in decision making process on the number of doses required for selected populations and to evaluate potential consequences of future vaccines. Sustaining high vaccine coverage is important to prevent nasopharyngeal colonization with vaccine serotypes and reduce vaccine type IPD via indirect protection. Continued surveillance is necessary to determine the impact of PCV13 and potential next generation pneumococcal vaccines.
Citation: M. Yildirim, P. Keskinocak, S. Pelton et al., Who is at risk of 13-valent conjugated pneumococcal vaccine failure?, Vaccine, https://doi.org/10.1016/j.vaccine.2019.12.060

