In Focus
‘C-Reactive Protein in Late-Onset Neonatal Sepsis’
Assessment of C-Reactive Protein Diagnostic Test Accuracy for Late-Onset Infection in Newborn Infants: A Systematic Review and Meta-analysis
Key Points
Question Is serum C-reactive protein level sufficiently accurate to aid the diagnosis of late-onset infection in newborn infants?
Findings In this systematic review and meta-analysis of 22 cohort studies (2255 infants) comparing the diagnostic test accuracy of serum C-reactive protein with microbiological culture, median specificity was 0.74 and pooled sensitivity was 0.62. Assuming a orwould miss 152 cases of infection and wrongly diagnose 156 cases.
Meaning The findings suggest that serum C-reactive protein level is not sufficiently accurate to aid diagnosis or to inform treatment decisions in infants with suspected late-onset infection.
Abstract
Importance Rapid and accurate diagnosis of late-onset infection in newborn infants could inform treatment decisions and avoid unnecessary administration of antibiotics.
Objective To compare the accuracy of serum C-reactive protein (CRP) with that of microbiological blood culture for diagnosing late-onset infection in newborns.
Study Selection Cohort and cross-sectional studies were included that compared the accuracy of serum CRP levels with microbiological culture results to diagnose late-onset (>72 hours after birth) infection in newborns (any gestational age) hospitalized after birth. Two reviewers assessed study eligibility. Among 10 394 records, 148 studies were assessed as full texts.
Main Outcomes and Measures The primary meta-analysis outcome was diagnostic test accuracy of serum CRP level taken at initial investigation of an infant with suspected late-onset infection. The median specificity (proportion of true-negative results) and calculated pooled sensitivity (proportion of true-positive results) were determined by generating hierarchical summary receiver characteristic operating curves.
Results In total, 22 studies with 2255 infants were included (sample size range, 11-590 infants). Participants in most studies were preterm (<37 weeks) or very low-birth weight (<1500 g) infants. Two studies additionally enrolled infants born at term. Most studies (14 of 16) used a prespecified CRP level cutoff for a “positive” index test (5-10 mg/L) and the culture of a pathogenic microorganism from blood as the reference standard. Risk of bias was low with independent assessment of index and reference tests. At median specificity (0.74), pooled sensitivity was 0.62 (95% CI, 0.50-0.72). Adding serum CRP level to the assessment of an infant with a 40% pretest probability of late-onset infection (the median for the included studies) generated posttest probabilities of 26% for a negative test result and 61% for a positive test result.
Conclusions and Relevance The findings suggest that determination of serum CRP level at initial evaluation of an infant with suspected late-onset infection is unlikely to aid early diagnosis or to select infants to undergo further investigation or treatment with antimicrobial therapy or other interventions.
(Jennifer Valeska Elli Brown, et al. JAMA Pediatr. Published online February 3, 2020. doi:10.1001/jamapediatrics.2019.5669)
C-Reactive Protein Testing in Late-Onset Neonatal Sepsis: Hazardous Waste!
Imagine for a moment you are counseling a patient with a suspicious mass and symptoms that are concerning but nonspecific. Although there are several possible diagnoses, one hangs unspoken between you and the patient—a diagnosis that carries significant morbidity and mortality. You propose an immediate biopsy. After all, a tissue diagnosis is the criterion standard, and this matter is urgent. The patient agrees, and as the patient begins to stand, you raise a hand and mention another test you would like to order. The patient is curious. “What are the benefits of this test?” You explain that the test involves sending a portion of the biopsied tissue to a separate part of the laboratory, where the pathology department will look for other markers of disease. Of course, the patient nods; that makes sense. “If this extra test result is negative, I will be okay, right?” “No,” you answer, “a negative test result does not mean the biopsy result will be negative.” The patient frowns. “Well, is it a bad sign if the test result is positive?” “Not necessarily,” you explain. “Many things can make the other test results abnormal, so we’ll still have to wait on the biopsy results. The biopsy is the key.” The patient sits down again, looking confused. “If the biopsy is so important, why are we wasting tissue on this other test?” “Well,” you glance helplessly toward the window then look back to your frowning patient. “It’s just what we’ve always done in these cases.”
This exchange is hypothetical, but in the case of C-reactive protein (CRP) for neonatal sepsis, it is all too real. The nonspecific signs of late-onset infection in infants, particularly those born preterm, combined with the high risk of morbidity and mortality have underscored the need for accurate diagnosis of neonatal sepsis.1 Culture of blood and other sterile sites is the criterion standard for neonatal sepsis. However, myriad ancillary laboratory tests have been suggested as potential biomarkers for neonatal sepsis; these include CRP, complete blood counts with differential, procalcitonin, a variety of interleukins, and presepsin.2 The value of those tests in the clinical management of suspected sepsis is questionable. In this issue of JAMA Pediatrics, Brown et al3 report their systematic review and meta-analysis of the sensitivity, specificity, and test accuracy of CRP for late-onset neonatal sepsis. They analyzed 22 studies including 2255 infants, the majority being 32 weeks or less gestational age or 1500 g or less birth weight. The median specificity of CRP was 0.74 and median sensitivity was 0.62. Assuming a cohort of 1000 preterm infants with a 20% prevalence rate of late-onset sepsis, this means that 76 cases of infection would be missed, and 208 infants would be incorrectly diagnosed as having sepsis—more than the number of infants with sepsis (200).
The poor sensitivity and specificity of CRP as a biomarker renders the test essentially useless. The poor sensitivity means that CRP levels cannot be used to prevent antimicrobial treatment; infants with suspected late-onset sepsis require empirical antibiotics while their cultures are incubating. The sensitivity of the CRP test may be lowest among infants with lower gestational age and birth weight, meaning that CRP performs the worst among infants with the highest risk for sepsis.4 In addition, the poor specificity means that CRP should not be used to make decisions regarding antibiotic duration when cultures are sterile because most abnormal CRP results are false-positives. The positive predictive value of CRP becomes truly abysmal as the prevalence rate of late-onset sepsis declines. For example, the prevalence rate of sepsis decreases to less than 5% by 30 weeks’ gestation, decreasing the positive predictive value to below 10%.5 For infants with birth weight higher than 1500 g, the positive predictive value is negligible. Unfortunately, treatment of “culture-negative” sepsis is both common and often driven by falsely positive ancillary laboratory testing, such as CRP.6
Proponents of CRP for the diagnosis of neonatal sepsis point to its excellent negative predictive value as a redeeming feature. However, the negative predictive value is driven more by the relatively low prevalence of late-onset sepsis than by the test characteristics of CRP. For example, by applying CRP level results to the diagnosis of late-onset sepsis in a large cohort of preterm infants 23 to 33 weeks’ gestation admitted to the Neonatal Research Network neonatal intensive care units,5 we calculated a negative predictive value of 95.5% (Table). However, when a fair coin pulled from a desk drawer is used on the same cohort, “tails”—arbitrarily chosen as a negative screening test—performs almost as well (91.5%). In any case, the poor sensitivity of CRP means that physicians are forced to treat the infant empirically regardless of the test result so as not to miss the meaningful number of septic infants with falsely negative CRPs. The negative predictive value of CRP is not clinically useful.
Table
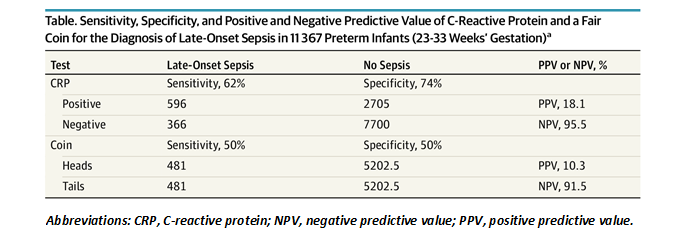
The unacceptable number of false-positive and false-negative results means that trusting CRP results is dangerous. The systematic use of CRP in the diagnosis of late-onset sepsis is not only hazardous, it is wasteful. A better use for the blood required to conduct the test would be to inoculate it into culture media. The criterion standard for late-onset sepsis is blood culture, and the accuracy of blood culture is driven directly by the volume of blood obtained for culture.7,8 The challenges of obtaining sufficient volume for culture in preterm infants have been well described.9 Why are we devoting between 0.2 and 0.5 mL of precious blood volume to CRP and its poor test characteristics, instead of optimizing our criterion standard? The potential harm from the systematic use of CRP—delay in empirical therapy, overtreatment of uninfected infants, and decreased sensitivity of blood culture because of the redirected blood volume—greatly outweighs the negligible benefit to clinical management. This applies not only to CRP but to complete blood counts with differential, procalcitonin, and other biomarkers.10-12
Neonatal sepsis remains a question in search of better answers, and we applaud the clinical investigators working toward a rapid, accurate assay that precludes the need for antibiotic exposure in low-risk infants. Such an assay would significantly advance our ability to avoid unnecessary antimicrobial therapy while still ensuring that no infant with sepsis is missed. However, as Brown et al3 so eloquently illustrate in their systematic review, CRP testing is far from being such an assay. Instead, CRP is an insensitive, nonspecific test that steals blood volume from the criterion standard culture. The continued use of CRP in the diagnosis of late-onset neonatal sepsis should be considered hazardous waste.
References
- Lancet. 2017;390(10104):1770-1780.
- Am J Perinatol. 2018;35(6):575-577.
- JAMA Pediatr. doi:10.1001/jamapediatrics.2019.5669
4. BMC Infect Dis. 2015;15:320. doi:10.1186/s12879-015-1069-7 - Pediatr Res. 2017;82(2):297-304.
- Lancet Infect Dis. 2016;16(10):1178-1184.
7.J Pediatr. 1996;129(2):275-278.
- Acta Paediatr. 2018;107(6):1043-1048.
9.Clin Microbiol Infect. 2019;S1198-743X(19)30533-6.
- J Pediatr. 2019;205:105-111.e2
- Eur J Pediatr. 2018;177(5):625-632.
- Intensive Care Med. 2011;37(5):747-762.
(Joseph B. Cantey, Charlene R. Bultmann, In JAMA Pediatr. Published online February 3, 2020. doi:10.1001/jamapediatrics.2019.5684)

