Vaccinology Update
Are we on the verge of developing an effective vaccine against atherosclerosis?
Cardiovascular diseases (CVD) constitute a heterogeneous group of heart and blood vessel disorders that together are the leading cause of death worldwide. Atherosclerosis, a disease of large- and medium-sized arteries characterised by lipid-rich atherosclerotic plaques, is the most common pathology of CVD. When plaques rupture or erode, Major Adverse Cardiovascular Events (MACE) ensue, including stroke and myocardial infarction (MI). The etiology of atherosclerosis is complex, with contributions from genetic, dietary, lifestyle, metabolic and immune components. However, the primary events involve accumulation of modified lipoproteins, especially low density lipoprotein (LDL) in the vessel walls, which then triggers a cascade of pro-inflammatory events. During atherogenesis, LDL accumulates in the artery wall, where it becomes oxidized, resulting in reactive aldehyde groups such as malonaldehyde (MDA). Scavenger receptor mediated uptake of oxLDL by macrophages result in foam cell formation. It is controversial whether foam cells are pro-inflammatory. Current therapies available for atherosclerosis, such as statins and Proprotein Convertase Subtilisin/Kexin type 9(PCSK9) inhibitors, decrease LDL cholesterol levels in the blood. Although these interventions are among the most successful prevention strategies known in medicine, significant residual risk persists due to ongoing inflammation.
Recently, immunomodulatory therapeutic approaches to combat atherosclerosis have been explored. Anti-inflammatory drugs and monoclonal antibodies (mAbs) that target the ongoing immune reaction have been tested in both pre-clinical and clinical studies. For example, in a large cohort of patients with coronary heart disease, the Canakinumab Anti-inflammatory thrombosis outcomes study (CANTOS) trial tested the effects of the anti-IL-1b mAb canakinumab that successfully reduced MACE. However, systemic suppression of IL-1b negatively impacted host defence, leaving subjects more susceptible to lethal and non-lethal infections.
Substantial evidence from experimental models and clinical studies has established the role of inflammation and immune effector mechanisms in the pathogenesis of atherosclerosis. Several stages of the disease are affected by host-mediated antigen-specific adaptive immune responses that play either protective or proatherogenic roles. Therefore, strategies to boost an anti-atherogenic humoral and T regulatory cell response are emerging as preventative or therapeutic strategies to lowering inflammatory residual risks. Vaccination holds promise as an efficient, durable and relatively inexpensive approach to induce protective adaptive immunity in atherosclerotic patients.
There are several issues that need further elucidation before an effective vaccine against it can be developed. For example, there is a need to identify the immune targets in pre-clinical and clinical investigations, proper evaluation of immunization strategies in animal models, clinical trials to examine the safety and efficacy of human atherosclerosis vaccines and strategies to improve and optimize vaccination in humans (antigen selection, formulation, dose and delivery).
Vaccination as a promising strategy to combat atherosclerosis
Vaccination involves an active immunization process that can induce a durable and highly specific anti-atherogenic adaptive immune response in the recipient. However, unlike vaccines for infectious diseases and cancer that aim to boost pro-inflammatory and lytic T cell response, atherosclerosis vaccine formulations need to induce immunological tolerance and/or functional neutralization to alleviate the inflammatory response. Fundamentally, there are two strategies: inducing B cell-dependent production of neutralizing antibodies that can either block protein function (eg: anti-PCSK9) to improve lipid profiles or may facilitate increased oxLDL uptake by phagocytosis and subsequent clearance from circulation (eg: anti-oxLDL); inducing a durable Treg or Tr1 response. Ideally, vaccination promises specific and long-term protection, is inexpensive and thus accessible to millions of people around the globe.
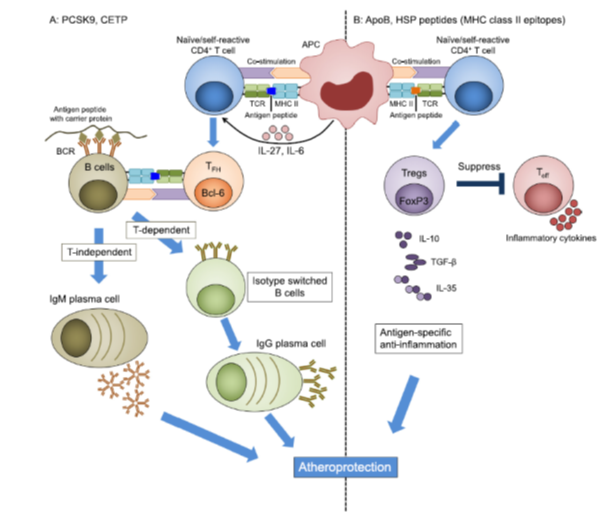
Fig. 1. Approaches to atherosclerosis vaccines. A: Antibody-based vaccines may target PCSK9, CETP or other proteins. The antigenic protein is engineered, possibly using acarrier prote in (tan). MHC-II-restricted T cell epitopes induce a CD4 T cell response that, in the presence of IL-6 and IL-27, can result in follicular helper TFH cells, characterized by the transcription factor Bcl6 (orange). In germinal centres, TFH cells provide help to B cells, induce antibody isotype switch from IgM to IgG and support the maturation of B cells to long-lived IgG-secreting plasma cells (green). Without T cell help, B cells can mature into IgM-producing plasma cells (tan). B: Self-reactive Tregs can be targeted when self- peptides are administered through an appropriate route and formulated in a suitable adjuvant. After vaccination, antigen-specific peptides are presented to naïve self-reactive T cells through MHC class II on APCs. This results in activation of antigen-specific Tregs. Activated Tregs suppress effector T cells by cell contact-dependent mechanisms and by secreting anti-inflammatory cytokines, such as IL-10, IL-35, and TGFb. ApoB: apolipoprotein B, APC: antigen-presenting cell, TFH: T follicular cell, Treg: regulatory T cell, Teff: T effector cell, MHC: major histocompatibility complex, TCR: T cell receptor. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Conclusions
A pivotal role of the adaptive immune system has now been firmly established in animal models of atherosclerosis, with strong correlative evidence emerging from clinical studies as well. These studies have provided ample motivation for the development of novel therapeutic interventions that target inflammation. Global immunosuppressive or anti-cytokine strategies can increase the risk of infections. Monoclonal antibodies to PCSK9 are approved, successful, but inconvenient for the patient and expensive for the health care system. siRNA to PCSK9 promises longer-lived responses of up to 9 months. Pre-clinical data suggests that immunization-induced humoral and cell-based adaptive immune responses can curb the progression of atherosclerosis. However, the antigen selection, vaccine design and the immunization regime need to be optimized before a human atherosclerosis vaccine may be developed. To achieve these goals, it is critical to have a better understanding of the human-specific molecular mechanisms underlying disease progression. Likely, the design and development of a preventative vaccine will differ substantially from that of a therapeutic vaccine.
Original citation: Payel Roy P, et al. Opportunities for an atherosclerosis vaccine: From mice to humans. Vaccines 2020; DOI: https://doi.org/10.1016/j.vaccine.2019.12.039

